id: 12479
question: Think about the magnetic force between the magnets in each pair. Which of the following statements is true?
choice: (A) The magnitude of the magnetic force is greater in Pair 2. (B) The magnitude of the magnetic force is the same in both pairs. (C) The magnitude of the magnetic force is greater in Pair 1.
context: The images below show two pairs of magnets. The magnets in different pairs do not affect each other. All the magnets shown are made of the same material, but some of them are different sizes and shapes.
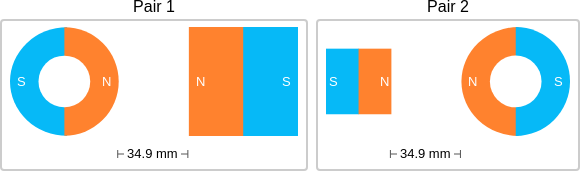
gold answer: C
wrong prediction:
Rationale:
- 0: Magnets can pull or push on each other without touching.
- 1: When magnets attract, they pull together.
- 2: When magnets repel, they push apart.
- 3: These pulls and pushes between magnets are called magnetic forces.
- 4: The strength of a force is called its magnitude.
- 5: The greater the magnitude of the magnetic force between two magnets, the more strongly the magnets attract or repel each other.
- 6: You can change the magnitude of a magnetic force between two magnets by using magnets of different sizes.
- 7: The magnitude of the magnetic force is greater when the magnets are larger.
- 8: Magnet sizes affect the magnitude of the magnetic force.
- 9: Imagine magnets that are the same shape and made of the same material.
- 10: The larger the magnets, the greater the magnitude of the magnetic force between them.
- 11: Magnet A is the same size in both pairs.
- 12: But Magnet B is larger in Pair 2 than in Pair 1.
- 13: So, the magnitude of the magnetic force is greater in Pair 2 than in Pair 1.
- 14: The answer is A.
id: 12497
question: Would you find the word which on a dictionary page with the following guide words? wide - wren
choice: (A) yes (B) no
gold answer: B
wrong prediction:
Rationale:
- 0: Guide words appear on each page of a dictionary.
- 1: They tell you the first word and last word on the page.
- 2: The other words on the page come between the guide words in alphabetical order.
- 3: To put words in alphabetical order, put them in order by their first letters.
- 4: If the first letters are the same, look at the second letters.
- 5: If the second letters are the same, look at the third letters, and so on.
- 6: If one word is shorter, and there are no more letters to compare, then the shorter word comes first in alphabetical order.
- 7: For example, be comes before bed.
- 8: Put the words in alphabetical order.
- 9: Since which is between the guide words wide - wren, it would be found on that page.
- 10: The answer is A.
id: 12546
question: Which solution has a higher concentration of purple particles?
choice: (A) Solution B (B) neither; their concentrations are the same (C) Solution A
context: The diagram below is a model of two solutions. Each purple ball represents one particle of solute.
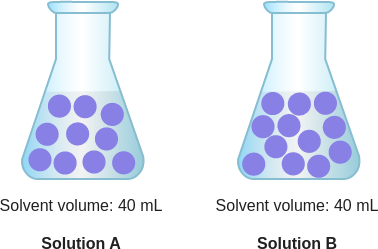
gold answer: A
wrong prediction:
Rationale:
- 0: A solution is made up of two or more substances that are completely mixed.
- 1: In a solution, solute particles are mixed into a solvent.
- 2: The solute cannot be separated from the solvent by a filter.
- 3: For example, if you stir a spoonful of salt into a cup of water, the salt will mix into the water to make a saltwater solution.
- 4: In this case, the salt is the solute.
- 5: The water is the solvent.
- 6: The concentration of a solute in a solution is a measure of the ratio of solute to solvent.
- 7: Concentration can be described in terms of particles of solute per volume of solvent.
- 8: concentration = particles of solute / volume of solvent In Solution A and Solution B, the purple particles represent the solute.
- 9: To figure out which solution has a higher concentration of purple particles, look at both the number of purple particles and the volume of the solvent in each container.
- 10: Use the concentration formula to find the number of purple particles per milliliter.
- 11: Solution A has more purple particles per milliliter.
- 12: So, Solution A has a higher concentration of purple particles.
- 13: The answer is C.
id: 12583
question: Which trait did Miocidaris have? Select the trait you can observe on the fossil.
choice: (A) a mostly orange body (B) a mushroom-shaped body (C) straight spines
context: This picture shows a fossil of an ancient animal called Miocidaris. Miocidaris lived in the ocean over 230,000,000 years ago.

gold answer: C
wrong prediction:
Rationale:
- 0: The way an organism looks or acts is called a trait.
- 1: Scientists use fossils to learn more about the traits of ancient organisms.
- 2: Fossils can preserve the remains of body parts and activities.
- 3: A fossil of a body part, such as a tail or a wing, can tell you what an organism looked like.
- 4: A fossil of an organism's activities, such as a burrow or a footprint, can tell you about the organism's behavior.
- 5: Here are three examples of fossils and the traits that you can observe from them: This is a fossil of an animal.
- 6: This fossil tells you that the animal had a spiral-shaped shell.
- 7: This is a fossil of a plant.
- 8: This fossil tells you that the plant had small leaves arranged in a branched pattern.
- 9: This is a fossil of an animal's footprint.
- 10: This fossil tells you that the animal could walk on land.
- 11: An organism's fossil may not show all of the organism's traits.
- 12: This is because most body parts are destroyed during fossil formation.
- 13: When an organism's body turns into a fossil, only a few body parts are usually preserved.
- 14: The answer is B.
id: 12679
question: Compare the average kinetic energies of the particles in each sample. Which sample has the higher temperature?
choice: (A) neither; the samples have the same temperature (B) sample A (C) sample B
context: The diagrams below show two pure samples of gas in identical closed, rigid containers. Each colored ball represents one gas particle. Both samples have the same number of particles.
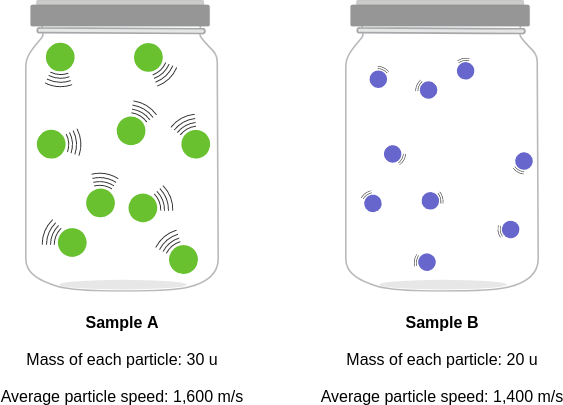
gold answer: B
wrong prediction:
Rationale:
- 0: The temperature of a substance depends on the average kinetic energy of the particles in the substance.
- 1: The higher the average kinetic energy of the particles, the higher the temperature of the substance.
- 2: The kinetic energy of a particle is determined by its mass and speed.
- 3: For a pure substance, the greater the mass of each particle in the substance and the higher the average speed of the particles, the higher their average kinetic energy.
- 4: The particles in both samples have the same average speed, but each particle in sample B has more mass than each particle in sample A.
- 5: So, the particles in sample B have a higher average kinetic energy than the particles in sample A.
- 6: Because the particles in sample B have the higher average kinetic energy, sample B must have the higher temperature.
- 7: The answer is C.
id: 12906
question: Think about the magnetic force between the magnets in each pair. Which of the following statements is true?
choice: (A) The magnitude of the magnetic force is smaller in Pair 1. (B) The magnitude of the magnetic force is the same in both pairs. (C) The magnitude of the magnetic force is smaller in Pair 2.
context: The images below show two pairs of magnets. The magnets in different pairs do not affect each other. All the magnets shown are made of the same material, but some of them are different sizes and shapes.
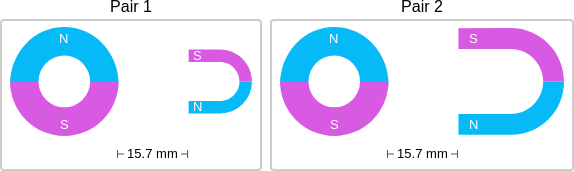
gold answer: A
wrong prediction:
Rationale:
- 0: Magnets can pull or push on each other without touching.
- 1: When magnets attract, they pull together.
- 2: When magnets repel, they push apart.
- 3: These pulls and pushes between magnets are called magnetic forces.
- 4: The strength of a force is called its magnitude.
- 5: The greater the magnitude of the magnetic force between two magnets, the more strongly the magnets attract or repel each other.
- 6: You can change the magnitude of a magnetic force between two magnets by using magnets of different sizes.
- 7: The magnitude of the magnetic force is smaller when the magnets are smaller.
- 8: Magnet sizes affect the magnitude of the magnetic force.
- 9: Imagine magnets that are the same shape and made of the same material.
- 10: The smaller the magnets, the smaller the magnitude of the magnetic force between them.
- 11: Magnet A is the same size in both pairs.
- 12: But Magnet B is smaller in Pair 2 than in Pair 1.
- 13: So, the magnitude of the magnetic force is smaller in Pair 2 than in Pair 1.
- 14: The answer is C.
id: 12959
question: Compare the average kinetic energies of the particles in each sample. Which sample has the higher temperature?
choice: (A) sample A (B) sample B (C) neither; the samples have the same temperature
context: The diagrams below show two pure samples of gas in identical closed, rigid containers. Each colored ball represents one gas particle. Both samples have the same number of particles.
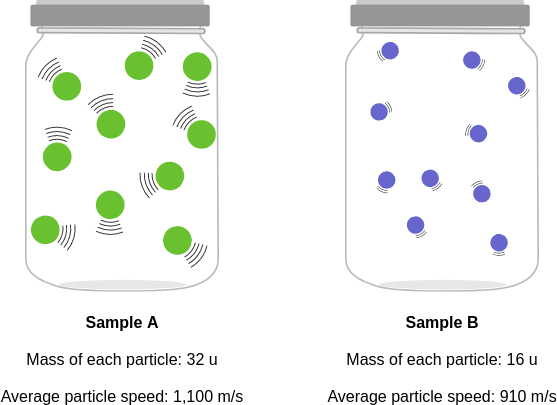
gold answer: A
wrong prediction:
Rationale:
- 0: The temperature of a substance depends on the average kinetic energy of the particles in the substance.
- 1: The higher the average kinetic energy of the particles, the higher the temperature of the substance.
- 2: The kinetic energy of a particle is determined by its mass and speed.
- 3: For a pure substance, the greater the mass of each particle in the substance and the higher the average speed of the particles, the higher their average kinetic energy.
- 4: Each particle in sample B has more mass than each particle in sample A.
- 5: The particles in sample B also have a higher average speed than the particles in sample A.
- 6: So, the particles in sample B have a higher average kinetic energy than the particles in sample A.
- 7: Because the particles in sample B have the higher average kinetic energy, sample B must have the higher temperature.
- 8: The answer is B.
id: 13035
question: Which word would you find on a dictionary page with the following guide words? sped - sworn
choice: (A) smash (B) stunt
gold answer: B
wrong prediction:
Rationale:
- 0: Guide words appear on each page of a dictionary.
- 1: They tell you the first word and last word on the page.
- 2: The other words on the page come between the guide words in alphabetical order.
- 3: To put words in alphabetical order, put them in order by their first letters.
- 4: If the first letters are the same, look at the second letters.
- 5: If the second letters are the same, look at the third letters, and so on.
- 6: If one word is shorter, and there are no more letters to compare, then the shorter word comes first in alphabetical order.
- 7: For example, be comes before bed.
- 8: Put the words in alphabetical order.
- 9: Since smash is between the guide words sped - sworn, it would be found on that page.
- 10: The answer is A.
id: 13042
question: What is the direction of this push?
choice: (A) away from the dad's hands (B) toward the dad's hands
context: A girl's dad pushes her bike with his hands, and she rides forward.

gold answer: A
wrong prediction:
Rationale:
- 0: One object can make another object move with a push or a pull.
- 1: The direction of a push is away from the object that is pushing.
- 2: The direction of a pull is toward the object that is pulling.
- 3: The dad pushes his daughter forward, and she rides toward the dad's hands.
- 4: The direction of the push is toward the dad's hands.
- 5: The answer is B.
id: 13144
question: Which solution has a higher concentration of purple particles?
choice: (A) Solution B (B) Solution A (C) neither; their concentrations are the same
context: The diagram below is a model of two solutions. Each purple ball represents one particle of solute.
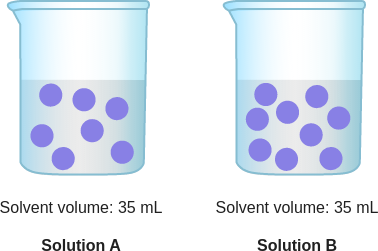
gold answer: A
wrong prediction:
Rationale:
- 0: A solution is made up of two or more substances that are completely mixed.
- 1: In a solution, solute particles are mixed into a solvent.
- 2: The solute cannot be separated from the solvent by a filter.
- 3: For example, if you stir a spoonful of salt into a cup of water, the salt will mix into the water to make a saltwater solution.
- 4: In this case, the salt is the solute.
- 5: The water is the solvent.
- 6: The concentration of a solute in a solution is a measure of the ratio of solute to solvent.
- 7: Concentration can be described in terms of particles of solute per volume of solvent.
- 8: concentration = particles of solute / volume of solvent In Solution A and Solution B, the purple particles represent the solute.
- 9: To figure out which solution has a higher concentration of purple particles, look at both the number of purple particles and the volume of the solvent in each container.
- 10: Use the concentration formula to find the number of purple particles per milliliter.
- 11: Solution A has more purple particles per milliliter.
- 12: So, Solution A has a higher concentration of purple particles.
- 13: The answer is B.