id: 3372
lecture: Magnets can pull or push on each other without touching. When magnets attract, they pull together. When magnets repel, they push apart. These pulls and pushes between magnets are called magnetic forces. The stronger the magnetic force between two magnets, the more strongly the magnets attract or repel each other. You can change the strength of a magnetic force between two magnets by changing the distance between them. The magnetic force is stronger when the magnets are closer together.
question: Think about the magnetic force between the magnets in each pair. Which of the following statements is true?
choice: (A) The magnetic force is stronger in Pair 2. (B) The magnetic force is stronger in Pair 1. (C) The strength of the magnetic force is the same in both pairs.
context: The images below show two pairs of magnets. The magnets in different pairs do not affect each other. All the magnets shown are made of the same material.
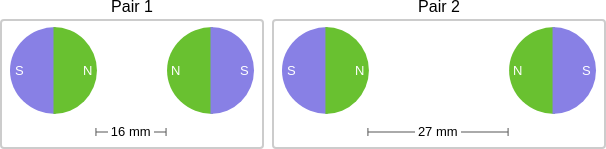
answer: B
Rationale:
- 0: Magnets can pull or push on each other without touching.
- 1: When magnets attract, they pull together.
- 2: When magnets repel, they push apart.
- 3: These pulls and pushes between magnets are called magnetic forces.
- 4: The stronger the magnetic force between two magnets, the more strongly the magnets attract or repel each other.
- 5: You can change the strength of a magnetic force between two magnets by changing the distance between them.
- 6: The magnetic force is stronger when the magnets are closer together.
- 7: Distance affects the strength of the magnetic force.
- 8: When magnets are closer together, the magnetic force between them is stronger.
- 9: The magnets in Pair 2 are closer together than the magnets in Pair 1.
- 10: So, the magnetic force is stronger in Pair 2 than in Pair 1.
- 11: The answer is A.
generated_skill: The missing skill in this demonstration is the ability to understand that the strength of a magnetic force is not solely determined by distance.
solution: Distance affects the strength of the magnetic force. When magnets are closer together, the magnetic force between them is stronger. The magnets in Pair 1 are closer together than the magnets in Pair 2. So, the magnetic force is stronger in Pair 1 than in Pair 2.
skill: Compare strengths of magnetic forces
wrong_step_idx: 9
id: 3453
lecture: The temperature of a substance depends on the average kinetic energy of the particles in the substance. The higher the average kinetic energy of the particles, the higher the temperature of the substance. The kinetic energy of a particle is determined by its mass and speed. For a pure substance, the greater the mass of each particle in the substance and the higher the average speed of the particles, the higher their average kinetic energy.
question: Compare the average kinetic energies of the particles in each sample. Which sample has the higher temperature?
choice: (A) sample A (B) neither; the samples have the same temperature (C) sample B
context: The diagrams below show two pure samples of gas in identical closed, rigid containers. Each colored ball represents one gas particle. Both samples have the same number of particles.
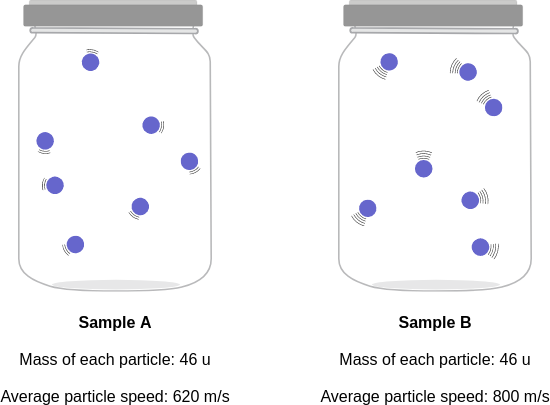
answer: C
Rationale:
- 0: The temperature of a substance depends on the average kinetic energy of the particles in the substance.
- 1: The higher the average kinetic energy of the particles, the higher the temperature of the substance.
- 2: The kinetic energy of a particle is determined by its mass and speed.
- 3: For a pure substance, the greater the mass of each particle in the substance and the higher the average speed of the particles, the higher their average kinetic energy.
- 4: Each particle in the two samples has the same mass, but the particles in sample A have a higher average speed than the particles in sample B.
- 5: So, the particles in sample A have a higher average kinetic energy than the particles in sample B.
- 6: Because the particles in sample A have the higher average kinetic energy, sample A must have the higher temperature.
- 7: The answer is A.
generated_skill: The missing skill in this query is analyzing the relationship between temperature and average kinetic energy of particles in a substance. The person incorrectly assumes that the substance with higher average kinetic energy has a higher temperature. However, the person fails to consider that temperature is a measure of the average kinetic energy of all the particles in the substance, not just one particle.
solution: Each particle in the two samples has the same mass, but the particles in sample B have a higher average speed than the particles in sample A. So, the particles in sample B have a higher average kinetic energy than the particles in sample A. Because the particles in sample B have the higher average kinetic energy, sample B must have the higher temperature.
skill: Identify how particle motion affects temperature and pressure
wrong_step_idx: 4
id: 3487
lecture:
question: Select the description that doesn't belong.
choice: (A) clever (B) foolish (C) wise (D) scared
context: N/A
answer: D
Rationale:
- 0: Wisdom is the quality of being wise.
- 1: A wise person is clever and knows what to do.
- 2: Foolish is the quality of being foolish.
- 3: A foolish person is scared and does things that may cause problems.
- 4: Scared is a description for a person who is afraid.
- 5: A person who is scared may run or hide.
- 6: Clever is a description for a person who is wise.
- 7: A clever person has quick, sharp intelligence.
- 8: The answer is C.
generated_skill: The missing skill in this scenario is the ability to identify and categorize descriptions based on their characteristics or qualities.
solution: Scared doesn't belong. Clever, foolish, and wise all describe how smart someone is.
skill: Select the words that don't belong
wrong_step_idx: 8
id: 3553
lecture: A solution is made up of two or more substances that are completely mixed. In a solution, solute particles are mixed into a solvent. The solute cannot be separated from the solvent by a filter. For example, if you stir a spoonful of salt into a cup of water, the salt will mix into the water to make a saltwater solution. In this case, the salt is the solute. The water is the solvent. The concentration of a solute in a solution is a measure of the ratio of solute to solvent. Concentration can be described in terms of particles of solute per volume of solvent. concentration = particles of solute / volume of solvent
question: Which solution has a higher concentration of yellow particles?
choice: (A) Solution B (B) neither; their concentrations are the same (C) Solution A
context: The diagram below is a model of two solutions. Each yellow ball represents one particle of solute.
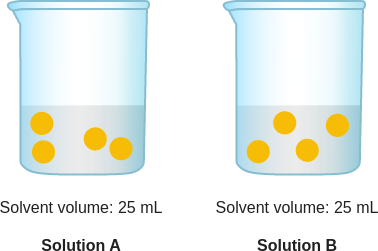
answer: B
Rationale:
- 0: A solution is made up of two or more substances that are completely mixed.
- 1: In a solution, solute particles are mixed into a solvent.
- 2: The solute cannot be separated from the solvent by a filter.
- 3: For example, if you stir a spoonful of salt into a cup of water, the salt will mix into the water to make a saltwater solution.
- 4: In this case, the salt is the solute.
- 5: The water is the solvent.
- 6: The concentration of a solute in a solution is a measure of the ratio of solute to solvent.
- 7: Concentration can be described in terms of particles of solute per volume of solvent.
- 8: concentration = particles of solute / volume of solvent In Solution A and Solution B, the yellow particles represent the solute.
- 9: To figure out which solution has a higher concentration of yellow particles, look at both the number of yellow particles and the volume of the solvent in each container.
- 10: Use the concentration formula to find the number of yellow particles per milliliter.
- 11: Solution A has more yellow particles per milliliter.
- 12: So, Solution A has a higher concentration of yellow particles.
- 13: The answer is C.
generated_skill: The missing skill in this case is understanding that concentration is determined by the ratio of solute to solvent, not just the number of particles.
solution: In Solution A and Solution B, the yellow particles represent the solute. To figure out which solution has a higher concentration of yellow particles, look at both the number of yellow particles and the volume of the solvent in each container. Use the concentration formula to find the number of yellow particles per milliliter. Solution A and Solution B have the same number of yellow particles per milliliter. So, their concentrations are the same.
skill: Compare concentrations of solutions
wrong_step_idx: 11
id: 3560
lecture: Solid, liquid, and gas are states of matter. Matter is anything that takes up space. Matter can come in different states, or forms. When matter is a solid, it has a definite volume and a definite shape. So, a solid has a size and shape of its own. Some solids can be easily folded, bent, or broken. A piece of paper is a solid. Also, some solids are very small. A grain of sand is a solid. When matter is a liquid, it has a definite volume but not a definite shape. So, a liquid has a size of its own, but it does not have a shape of its own. Think about pouring juice from a bottle into a cup. The juice still takes up the same amount of space, but it takes the shape of the bottle. Some liquids are thicker than others. Honey and milk are both liquids. But pouring honey takes more time than pouring milk. When matter is a gas, it does not have a definite volume or a definite shape. A gas expands, or gets bigger, until it completely fills a space. A gas can also get smaller if it is squeezed into a smaller space. Many gases are invisible. The oxygen you breathe is a gas. The helium in a balloon is also a gas.
question: Is a juice pop a solid, a liquid, or a gas?
choice: (A) a liquid (B) a solid (C) a gas
context: N/A

answer: B
Rationale:
- 0: Solid, liquid, and gas are states of matter.
- 1: Matter is anything that takes up space.
- 2: Matter can come in different states, or forms.
- 3: When matter is a solid, it has a definite volume and a definite shape.
- 4: So, a solid has a size and shape of its own.
- 5: Some solids can be easily folded, bent, or broken.
- 6: A piece of paper is a solid.
- 7: Also, some solids are very small.
- 8: A grain of sand is a solid.
- 9: When matter is a liquid, it has a definite volume but not a definite shape.
- 10: So, a liquid has a size of its own, but it does not have a shape of its own.
- 11: Think about pouring juice from a bottle into a cup.
- 12: The juice still takes up the same amount of space, but it takes the shape of the bottle.
- 13: Some liquids do not pour as easily as others.
- 14: Honey and milk are both liquids.
- 15: But pouring honey takes more time than pouring milk.
- 16: When matter is a gas, it does not have a definite volume or a definite shape.
- 17: A gas expands, or gets bigger, until it completely fills a space.
- 18: A gas can also get smaller if it is squeezed into a smaller space.
- 19: Many gases are invisible.
- 20: Air is a gas.
- 21: A juice pop is a liquid.
- 22: A liquid takes the shape of any container it is in.
- 23: If you squeeze a juice pop, it will take the shape of a bottle-shaped container.
- 24: But the juice pop will still take up the same amount of space.
- 25: The answer is A.
generated_skill: The missing skill in this case is the ability to differentiate between different states of matter (solid, liquid, gas) and understand their characteristics. In the wrong rationale, there is confusion between the properties of liquids and solids, leading to the incorrect conclusion that a juice pop is a liquid. The correct understanding is that a juice pop is a solid because it has a definite shape and size, and it does not take the shape of the container it is in.
solution: A juice pop is a solid. A solid has a size and shape of its own. If you take a juice pop out of the freezer, it will melt. While a juice pop is frozen, though, it is a solid.
skill: Identify and sort solids, liquids, and gases
wrong_step_idx: 21
id: 3582
lecture: Guide words appear on each page of a dictionary. They tell you the first word and last word on the page. The other words on the page come between the guide words in alphabetical order. To put words in alphabetical order, put them in order by their first letters. If the first letters are the same, look at the second letters. If the second letters are the same, look at the third letters, and so on.
question: Would you find the word long on a dictionary page with the following guide words? level - lion
choice: (A) yes (B) no
context: N/A
answer: B
Rationale:
- 0: Guide words appear on each page of a dictionary.
- 1: They tell you the first word and last word on the page.
- 2: The other words on the page come between the guide words in alphabetical order.
- 3: To put words in alphabetical order, put them in order by their first letters.
- 4: If the first letters are the same, look at the second letters.
- 5: If the second letters are the same, look at the third letters, and so on.
- 6: If one word is shorter, and there are no more letters to compare, then the shorter word comes first in alphabetical order.
- 7: For example, be comes before bed.
- 8: Put the words in alphabetical order.
- 9: Since long is between the guide words level - lion, it would be found on that page.
- 10: The answer is A.
generated_skill: The missing skill in this case is understanding how to determine the alphabetical order of words using guide words in a dictionary.
solution: Put the words in alphabetical order. Since long is not between the guide words level - lion, it would not be found on that page.
skill: Use guide words
wrong_step_idx: 9
id: 3591
lecture: In a solution, solute particles move and spread throughout the solvent. The diagram below shows how a solution can change over time. Solute particles move from the area where they are at a higher concentration to the area where they are at a lower concentration. This movement happens through the process of diffusion. As a result of diffusion, the concentration of solute particles becomes equal throughout the solution. When this happens, the solute particles reach equilibrium. At equilibrium, the solute particles do not stop moving. But their concentration throughout the solution stays the same. Membranes, or thin boundaries, can divide solutions into parts. A membrane is permeable to a solute when particles of the solute can pass through gaps in the membrane. In this case, solute particles can move freely across the membrane from one side to the other. So, for the solute particles to reach equilibrium, more particles will move across a permeable membrane from the side with a higher concentration of solute particles to the side with a lower concentration. At equilibrium, the concentration on both sides of the membrane is equal.
question: Complete the text to describe the diagram. Solute particles moved in both directions across the permeable membrane. But more solute particles moved across the membrane (). When there was an equal concentration on both sides, the particles reached equilibrium.
choice: (A) to the right than to the left (B) to the left than to the right
context: The diagram below shows a solution with one solute. Each solute particle is represented by a pink ball. The solution fills a closed container that is divided in half by a membrane. The membrane, represented by a dotted line, is permeable to the solute particles. The diagram shows how the solution can change over time during the process of diffusion.
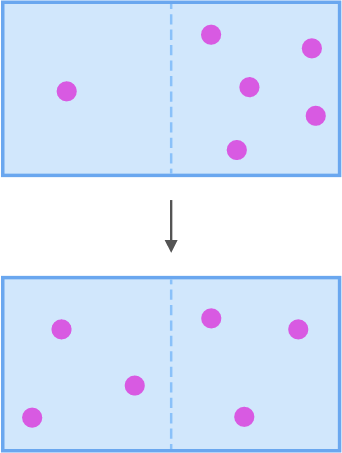
answer: B
Rationale:
- 0: In a solution, solute particles move and spread throughout the solvent.
- 1: The diagram below shows how a solution can change over time.
- 2: Solute particles move from the area where they are at a higher concentration to the area where they are at a lower concentration.
- 3: This movement happens through the process of diffusion.
- 4: As a result of diffusion, the concentration of solute particles becomes equal throughout the solution.
- 5: When this happens, the solute particles reach equilibrium.
- 6: At equilibrium, the solute particles do not stop moving.
- 7: But their concentration throughout the solution stays the same.
- 8: Membranes, or thin boundaries, can divide solutions into parts.
- 9: A membrane is permeable to a solute when particles of the solute can pass through gaps in the membrane.
- 10: In this case, solute particles can move freely across the membrane from one side to the other.
- 11: So, for the solute particles to reach equilibrium, more particles will move across a permeable membrane from the side with a higher concentration of solute particles to the side with a lower concentration.
- 12: At equilibrium, the concentration on both sides of the membrane is equal.
- 13: Look at the diagram again.
- 14: It shows you how the solution changed during the process of diffusion.
- 15: Before the solute particles reached equilibrium, there were 5 solute particles on the left side of the membrane and 3 solute particles on the right side of the membrane.
- 16: When the solute particles reached equilibrium, there were 4 solute particles on each side of the membrane.
- 17: There was 1 more solute particle on the right side of the membrane than before.
- 18: So, for the solute particles to reach equilibrium, more solute particles must have moved across the membrane to the right than to the left.
- 19: The answer is A.
generated_skill: The missing skill is accurately interpreting and analyzing the data presented in the diagram.
solution: Look at the diagram again. It shows you how the solution changed during the process of diffusion. Before the solute particles reached equilibrium, there was 1 solute particle on the left side of the membrane and 5 solute particles on the right side of the membrane. When the solute particles reached equilibrium, there were 3 solute particles on each side of the membrane. There were 2 more solute particles on the left side of the membrane than before. So, for the solute particles to reach equilibrium, more solute particles must have moved across the membrane to the left than to the right.
skill: Diffusion across membranes
wrong_step_idx: 15
id: 3610
lecture: During peer review, you read and respond to a fellow student's writing. While there are many methods and strategies that you can use for reviewing a text, it is generally helpful to frame your suggestions in concrete and constructive ways and to consider the following areas for revision: Ideas and development: Does the writer express a clear idea and develop it with evidence, examples, or analysis? Organization: Does the writer order ideas in a clear, logical way so that they build on one another and are easy to follow? Voice: Does the writer maintain an appropriate voice, such as a formal and objective voice in an academic essay or an engaging and expressive voice in a narrative essay? Sentence fluency: Does the writer use sentences that vary in structure and length to create a sense of rhythm and flow within and between sentences, or does the writing sound choppy, rambling, or repetitive? Word choice: Does the writer use words accurately and precisely to create clear, effective, and engaging writing? Grammar and mechanics: Does the writer follow appropriate conventions, using accurate spelling, punctuation, and grammar to create writing that is correct and easy to read?
question: Read the following excerpt from a student essay. How can the writer best improve his or her grammar and mechanics? In Great Expectations by Charles Dickens, Pip, a young orphan, is sent by his sister to the home of Miss Havisham, a wealthy, eccentric woman. There he meets Estella, a beautiful girl whom he falls hopelessly in love with. On one visit to Miss Havisham's, Pip encounters a boy who challenges him to a fight; wanting to impress Estella, he hits the boy. "I never have been so surprised in my life as I was when I let out the first blow and saw him lying on his back, looking up at me with a bloody nose", Pip recounts. Estella seems delighted and says to Pip "Come here! You may kiss me if you like".
choice: (A) by fixing run-on sentences (B) by punctuating quotations correctly (C) by using semicolons correctly
context: N/A
answer: B
Rationale:
- 0: During peer review, you read and respond to a fellow student's writing.
- 1: While there are many methods and strategies that you can use for reviewing a text, it is generally helpful to frame your suggestions in concrete and constructive ways and to consider the following areas for revision: Ideas and development: Does the writer express a clear idea and develop it with evidence, examples, or analysis?
- 2: Organization: Does the writer order ideas in a clear, logical way so that they build on one another and are easy to follow?
- 3: Voice: Does the writer maintain an appropriate voice, such as a formal and objective voice in an academic essay or an engaging and expressive voice in a narrative essay?
- 4: Sentence fluency: Does the writer use sentences that vary in structure and length to create a sense of rhythm and flow within and between sentences, or does the writing sound choppy, rambling, or repetitive?
- 5: Word choice: Does the writer use words accurately and precisely to create clear, effective, and engaging writing?
- 6: Grammar and mechanics: Does the writer follow appropriate conventions, using accurate spelling, punctuation, and grammar to create writing that is correct and easy to read?
- 7: The writer could best improve his or her grammar and mechanics by using semicolons correctly.
- 8: For example, the writer could use semicolons before the conjunctive adverb therefore in the underlined sentence to indicate that the two clauses are related.
- 9: In Great Expectations by Charles Dickens, Pip, a young orphan, is sent by his sister to the home of Miss Havisham, a wealthy, eccentric woman.
- 10: There he meets Estella, a beautiful girl whom he falls hopelessly in love with.
- 11: On one visit to Miss Havisham's, Pip encounters a boy who challenges him to a fight; wanting to impress Estella, he hits the boy.
- 12: "I never have been so surprised in my life as I was when I let out the first blow and saw him lying on his back, looking up at me with a bloody nose", Pip recounts.
- 13: Estella seems delighted and says to Pip "Come here!
- 14: You may kiss me if you like".
- 15: The answer is C.
generated_skill: The missing skill based on the wrong rationale steps is the correct use and punctuation of quotations. The writer is incorrectly punctuating the quotation in the sentence, "Come here! You may kiss me if you like". The correct punctuation should be: "Come here! You may kiss me if you like."
solution: The writer could best improve his or her grammar and mechanics by punctuating quotations correctly. For example, the writer could revise the underlined text by placing periods and commas inside the quotation marks and by using a comma before the second quotation. In Great Expectations by Charles Dickens, Pip, a young orphan, is sent by his sister to the home of Miss Havisham, a wealthy, eccentric woman. There he meets Estella, a beautiful girl whom he falls hopelessly in love with. On one visit to Miss Havisham's, Pip encounters a boy who challenges him to a fight; wanting to impress Estella, he hits the boy. "I never have been so surprised in my life as I was when I let out the first blow and saw him lying on his back, looking up at me with a bloody nose", Pip recounts. Estella seems delighted and says to Pip "Come here! You may kiss me if you like".
skill: Suggest appropriate revisions
wrong_step_idx: 7
id: 3619
lecture: Chemical changes and physical changes are two common ways matter can change. In a chemical change, the type of matter changes. The types of matter before and after a chemical change are always different. Burning a piece of paper is a chemical change. When paper gets hot enough, it reacts with oxygen in the air and burns. The paper and oxygen change into ash and smoke. In a physical change, the type of matter stays the same. The types of matter before and after a physical change are always the same. Cutting a piece of paper is a physical change. The cut pieces are still made of paper. A change of state is a type of physical change. For example, ice melting is a physical change. Ice and liquid water are made of the same type of matter: water.
question: Complete the sentence. A firework exploding is a ().
choice: (A) chemical change (B) physical change
context: N/A
answer: A
Rationale:
- 0: Chemical changes and physical changes are two common ways matter can change.
- 1: In a chemical change, the type of matter changes.
- 2: The types of matter before and after a chemical change are always different.
- 3: Burning a piece of paper is a chemical change.
- 4: When paper gets hot enough, it reacts with oxygen in the air and burns.
- 5: The paper and oxygen change into ash and smoke.
- 6: In a physical change, the type of matter stays the same.
- 7: The types of matter before and after a physical change are always the same.
- 8: Cutting a piece of paper is a physical change.
- 9: The cut pieces are still made of paper.
- 10: A change of state is a type of physical change.
- 11: For example, ice melting is a physical change.
- 12: Ice and liquid water are made of the same type of matter: water.
- 13: A firework exploding is a change of state.
- 14: So, it is a physical change.
- 15: The firework breaks into pieces of itself.
- 16: They are still made of the same type of matter.
- 17: The answer is B.
generated_skill: The missing skill is the ability to recognize that a firework exploding is a chemical change, not a physical change.
solution: A firework exploding is a chemical change. During the explosion, the type of matter in the firework changes. This change produces sound, heat, and light.
skill: Identify physical and chemical changes
wrong_step_idx: 13
id: 3625
lecture: There are more than 100 different chemical elements, or types of atoms. Chemical elements make up all of the substances around you. A substance may be composed of one chemical element or multiple chemical elements. Substances that are composed of only one chemical element are elementary substances. Substances that are composed of multiple chemical elements bonded together are compounds. Every chemical element is represented by its own atomic symbol. An atomic symbol may consist of one capital letter, or it may consist of a capital letter followed by a lowercase letter. For example, the atomic symbol for the chemical element boron is B, and the atomic symbol for the chemical element chlorine is Cl. Scientists use different types of models to represent substances whose atoms are bonded in different ways. One type of model is a ball-and-stick model. The ball-and-stick model below represents a molecule of the compound boron trichloride. In a ball-and-stick model, the balls represent atoms, and the sticks represent bonds. Notice that the balls in the model above are not all the same color. Each color represents a different chemical element. The legend shows the color and the atomic symbol for each chemical element in the substance.
question: Complete the statement. Ozone is ().
choice: (A) a compound (B) an elementary substance
context: The model below represents a molecule of ozone. zone gas in the atmosphere protects living things on Earth from some of the Sun's harmful rays.
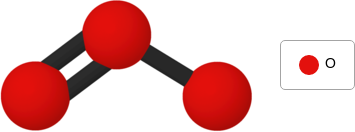
answer: B
Rationale:
- 0: There are more than 100 different chemical elements, or types of atoms.
- 1: Chemical elements make up all of the substances around you.
- 2: A substance may be composed of one chemical element or multiple chemical elements.
- 3: Substances that are composed of only one chemical element are elementary substances.
- 4: Substances that are composed of multiple chemical elements bonded together are compounds.
- 5: Every chemical element is represented by its own atomic symbol.
- 6: An atomic symbol may consist of one capital letter, or it may consist of a capital letter followed by a lowercase letter.
- 7: For example, the atomic symbol for the chemical element boron is B, and the atomic symbol for the chemical element chlorine is Cl.
- 8: Scientists use different types of models to represent substances whose atoms are bonded in different ways.
- 9: One type of model is a ball-and-stick model.
- 10: The ball-and-stick model below represents a molecule of the compound boron trichloride.
- 11: In a ball-and-stick model, the balls represent atoms, and the sticks represent bonds.
- 12: Notice that the balls in the model above are not all the same color.
- 13: Each color represents a different chemical element.
- 14: The legend shows the color and the atomic symbol for each chemical element in the substance.
- 15: Use the model to determine whether ozone is an elementary substance or a compound.
- 16: Step 1: Interpret the model.
- 17: .
- 18: Use the legend to determine the chemical element represented by each color.
- 19: The colors and atomic symbols from the legend are shown in the table below.
- 20: The table also includes the names of the chemical elements represented in the model.
- 21: You can see from the model that a molecule of ozone is composed of three oxygen atoms bonded together.
- 22: Step 2: Determine whether the substance is an elementary substance or a compound.
- 23: You know from Step 1 that ozone is composed of two chemical elements: oxygen and a special element that is not found in nature.
- 24: This special element is represented by the color red in the model.
- 25: So, ozone is composed of two chemical elements bonded together.
- 26: Substances that are composed of multiple chemical elements bonded together are compounds.
- 27: So, ozone is a compound.
- 28: The answer is A.
generated_skill: The missing skill in this scenario is interpreting the information provided by the legend in order to determine the chemical elements represented in the model.
solution: Use the model to determine whether ozone is an elementary substance or a compound. Step 1: Interpret the model. In the ball-and-stick model shown above, all of the balls are the same color: . The legend shows that red represents the chemical element with the atomic symbol O. O is the atomic symbol for the chemical element oxygen. You can see from the model that a molecule of ozone is composed of three oxygen atoms bonded together. Step 2: Determine whether the substance is an elementary substance or a compound. You know from Step 1 that ozone is composed of only one chemical element. So, ozone is an elementary substance.
skill: Classify elementary substances and compounds using models
wrong_step_idx: 23